Sensitization (ISO 10993-10)
Sensitization testing is used to assess the potential of chemicals and medical devices to cause a delayed hypersensitivity reaction following a single or repeated exposure to the body. Sensitization is one of the three most common biocompatibility tests required to ensure the safety of medical devices.
"Tests for irritation and skin sensitization", part ten of the Biological evaluation of medical devices standards (ISO 10993-10), gives out the general considerations that should be taken into account when evaluating the potential of inducing sensitizing of a medical device. It outlines the procedure of studies to assess the potential of medical devices and their constituent materials to induce irritation and skin sensitization.
General Procedures of the Sensitization Test
· Exposing the animals to the test materials or extracts across multiple induction phases and a single challenge phase;
· Observing the challenge sites for about 24 and 48 hours after the removal of the test materials or extracts and evaluating skin sensitization reactions.
Sensitization reactions are noted by observing erythema and/or edema as it interacts with the body's immune system.
STEMart offers several methods as in vivo and in vitro assays for sensitization biocompatibility testing following the biocompatibility guidelines modified for medical devices. The testing is designed and performed based on the route of exposure to the body and acceptance of the notified body.
Sensitization Testing Capabilities
Two types of tests have been developed: an adjuvant test in which sensitization is potentiated by the injection of Freund's Complete Adjuvant (FCA), and non-adjuvant tests.
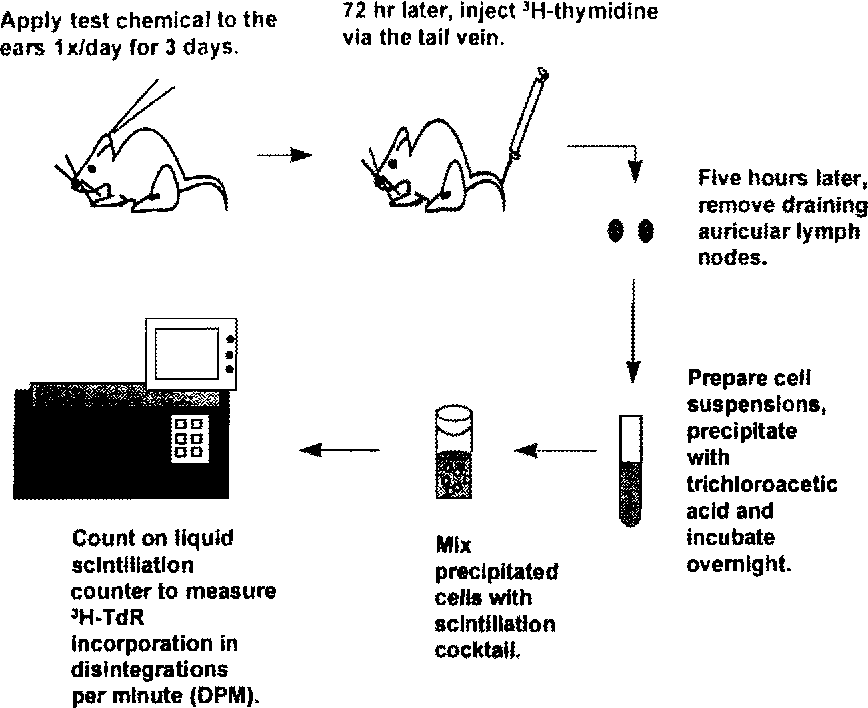
· Local Lymph Node Assay (LLNA)
LLNA studies the induction period of skin sensitization and delivers quantitative data for dose-response assessment. LLNA is an in vivo method that will not eliminate the use of animals but have the potential to reduce the number of animals required for this purpose and cause less pain and distress. LLNA does not require that challenge-induced dermal hypersensitivity reactions be elicited or the use of an adjuvant.

Fig. 1 The local lymph node assay method. (Gerberick, 2000)
· Guinea Pig Maximization Test (GPMT)
The GPMT is an in vivo test to screen for substances that cause human skin sensitization. The test animals are put together with an adjuvant, exposed intradermally to the test articles to enhance the immune reaction.
The Buehler Assay is a non-adjuvant in vivo test to screen for substances that cause human skin sensitization (i.e. allergens). In the test, Guinea pigs are exposed to a high dose of the substance, followed by a challenge dose, which is the highest dose that does not cause irritation.
· In Vitro Sensitization
We offer three-tiered in vitro skin sensitization tests: Direct Peptide Reactivity Assay (DPRA); ARE-Nrf2 Luciferase Test (KeratinoSens); Human Cell Line Activation Test (hCLAT)
If you have additional questions about Sensitization Testing or would like to find out more about our services, please feel free to contact us.
Reference
· Gerberick, G. Frank, et al. "Local lymph node assay: validation assessment for regulatory purposes." American Journal of Contact Dermatitis 11.1 (2000): 3-18.
- Whats New
- Shopping
- Wellness
- Sports
- Theater
- Religion
- Party
- Networking
- Music
- Literature
- Art
- Health
- Games
- Food
- Drinks
- Fitness
- Gardening
- Dance
- Causes
- Film
- Crafts
- Other/General
- Cricket
- Grooming
- Technology

