Europe Prophylaxis of Organ Rejection Market report has been prepared by considering several fragments of the present and upcoming market scenario. The market insights gained through this market research analysis report facilitates more clear understanding of the market landscape, issues that may interrupt in the future, and ways to position definite brand excellently. It consists of most-detailed market segmentation, thorough analysis of major market players, trends in consumer and supply chain dynamics, and insights about new geographical markets. The market insights covered in Europe Prophylaxis of Organ Rejection report simplifies managing marketing of goods and services effectively.
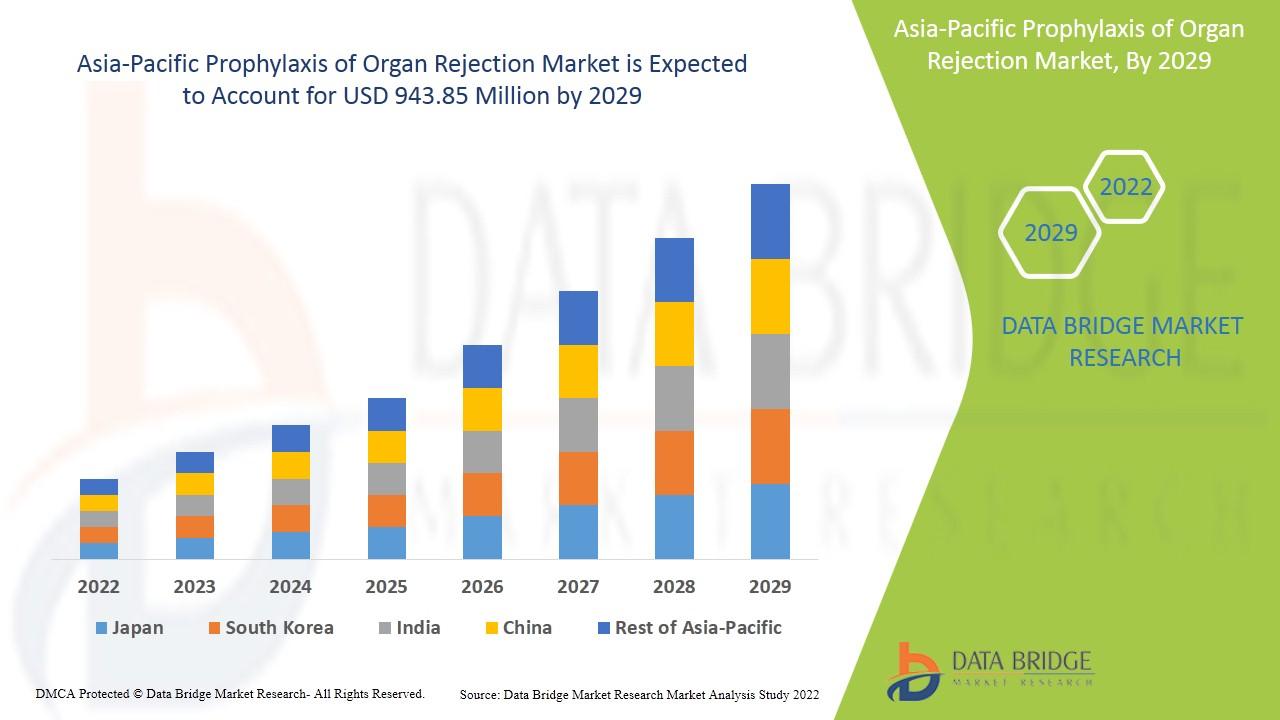
Europe prophylaxis of organ rejection is expected to gain market growth in the forecast period of 2021 to 2029. Data Bridge Market Research analyses that the Europe market is growing with a CAGR of 3.4% in the forecast period of 2022 to 2029 and is expected to reach USD 1,087.24 million by 2029 from USD 839.28 million in 2021.
Download Sample PDF Copy of this Report to understand structure of the complete report (Including Full TOC, Table & Figures) @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=europe-prophylaxis-of-organ-rejection-market
Market Overview:
The driving factors responsible for the growth of the North America prophylaxis of organ rejection market are the increased prevalence of organ transplantation, rise in surgical procedures, rise in recipient population and product launches. However, the factors that are expected to restrain the market are the rise in the cost of the transplantation procedure, lack of awareness about organ transplantation and the risks included while the patient receives the transplanted organ.
The major companies providing the Europe prophylaxis of organ rejection are Hansa Biopharma, Concord Biotech, Panacea Biotec, WOCKHARDT, SEBELA PHARMACEUTICALS, Accord-UK Ltd, Veloxis Pharmaceuticals, Inc, Astellas Pharma Inc, Novartis AG, Bristol-Myers Squibb Company, Dr Reddy’s Laboratories, Ltd, Viatris Inc, Strides Pharma Science Limited, Glenmark, Biocon, Pfizer Inc, Hikma Pharmaceuticals PLC, AbbVie Inc, Amneal Pharmaceuticals LLC, Zydus Pharmaceuticals, Inc., CSL Behring among others.
Europe Prophylaxis of Organ Rejection Market Country Level Analysis
Europe prophylaxis of organ rejection market is analysed and market size information is provided by cause, treatment, route of administration, organ, patient type, end user and distribution channel.
The countries covered in the Europe prophylaxis of organ rejection market report are the U.K., Germany, France, Spain, Italy, Netherlands, Switzerland, Russia, Belgium, Turkey and Rest of Europe.
Europe is expected to grow with the CAGR in the forecasted periods as in the growing R&D activities in the pulmonology segment. Germany is expected to dominate in the market in the Europe market. Germany is one of the leading countries due to the presence of major market players and increased technological advancements of Europe prophylaxis of organ rejection in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of Europe brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Browse Full Report Along with Facts and Figures @ https://www.databridgemarketresearch.com/reports/europe-prophylaxis-of-organ-rejection-market
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Check Complete Table of Contents with List of Table and Figures @ https://www.databridgemarketresearch.com/toc/?dbmr=europe-prophylaxis-of-organ-rejection-market
Browse Trending Reports:
https://www.databridgemarketresearch.com/reports/north-america-prophylaxis-of-organ-rejection-market
About Data Bridge Market Research:
An absolute way to predict what the future holds is to understand the current trend! Data Bridge Market Research presented itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are committed to uncovering the best market opportunities and nurturing effective information for your business to thrive in the marketplace. Data Bridge strives to provide appropriate solutions to complex business challenges and initiates an effortless decision-making process. Data Bridge is a set of pure wisdom and experience that was formulated and framed in 2015 in Pune.
Data Bridge Market Research has more than 500 analysts working in different industries. We have served more than 40% of the Fortune 500 companies globally and have a network of more than 5,000 clients worldwide. Data Bridge is an expert in creating satisfied customers who trust our services and trust our hard work with certainty. We are pleased with our glorious 99.9% customer satisfaction rating.
Contact Us: -
Data Bridge Market Research
US: +1 888 387 2818
United Kingdom: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: – [email protected]