Neuroinflammation
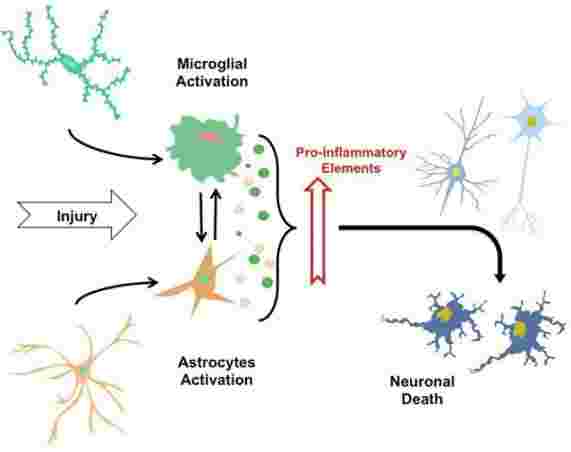
Neuroinflammation Overview
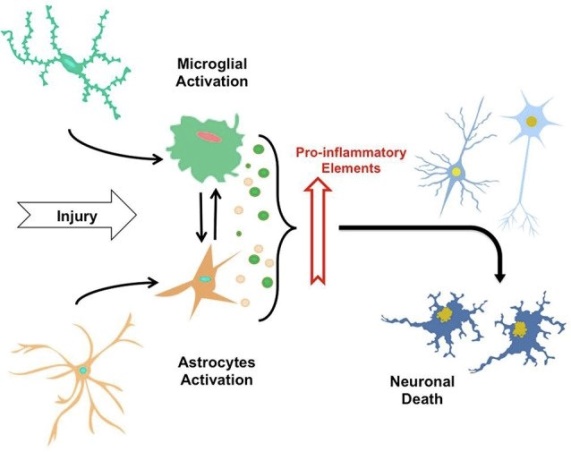
Chronic neuroinflammation is an important area of research because it plays an important role in common neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis disease (ALS) and multiple sclerosis (MS). In general, these diseases are characterized by an initial acute neuroinflammatory response that does not eliminate the pathogen. This is followed by a self-reproductive cascade of pathogenic events that cause chronic neuritis. Inflammation in the brain is induced by activated microglia and reactive astrocytes. It is known that glial cells play a role in both neuroprotection and neurodegenerative degeneration, so their role in neuroinflammation has become a research hotspot. Microglia are responsible for immune surveillance in the brain, which are immunologically active and phagocytic cells reside in the central nervous system. Astrocytes control blood flow and extracellular neurotransmitter levels to ensure that the microenvironment is best suited for neurological function. In the absence of irritation or injury, neurotransmitters, neurotrophic factors, anti-inflammatory cytokines, and intercellular contact immunosuppressive glial cells. However, when the irritation or injury exists, extracellular concentration of potassium, ATP or adenosine is increased, microglia and astrocytes are stimulated to release a strong mixture of pro-inflammatory mediators.
Mechanism of Neuroinflammation
The most common neuroinflammatory study is inflammation caused by glial cells, which are another major cell type in the nervous system other than neurons, accounting for 70% of the total number of cells in the central nervous system. Glial cells mainly include microglia, astrocytes, and oligodendrocytes. The traditional view is that glial cells act only as supporting cells for neurons and act to stabilize the network of neurons. However, there is increasing evidence that glial cells also play an important role in maintaining the ionic environment of neurons, regulating neural signaling, controlling neurotransmitter uptake, and assisting recovery after neuronal damage. With the continuous recognition of their functional diversity, such non-neuronal cells have gradually become the focus of research in the field of neuroscience. The current study found that many diseases in the field of neuroscience are closely related to the activation of glial cells, such as multiple sclerosis, amyotrophic lateral sclerosis, Huntington's disease, Alzheimer's disease, and Parkinson's disease. The common feature is the occurrence of a neuroinflammatory reaction. During the disease, microglia can release many inflammatory mediators, such as inflammatory cytokines, cell adhesion molecules, and chemokines, and can accelerate the progression of the disease. Therefore, this inflammatory response mediated by glial cells may be one of the mechanisms by which these diseases occur or worsen. Recently, there is increasing evidence that this glial cell-mediated neuroinflammatory response also plays an important role in the regulation of pain under pathological conditions. The generation and development of neuroinflammation have become a hot research topic in neurodegenerative diseases such as Parkinson's disease. According to previous studies, neuroinflammation mediates the progressive death of dopamine neurons in PD. The occurrence of neuroinflammation in PD mainly includes inflammatory events such as microglia activation, astrocyte activation, blood-brain barrier damage and T lymphocyte infiltration. Further, these central/peripheral immune cells interact through a variety of mechanisms to produce pro-inflammatory cytokines to amplify inflammatory signals, and produce neurotoxins that act directly on dopamine neurons, which ultimately leads to the death of dopamine neurons. Activation of immunogenic glial cells in the central nervous system is a major feature of neuroinflammation, including microglia and astrocyte activation. At the autopsy of PD patients, activated microglia (MHC-II positive) was found in the substantia nigra and striatum, suggesting microglia activation during PD development. In recent years, PK11195 has been used to label microglia in living brains, and positron emission tomography (PET-CT) has been used to confirm that microglia are activated in the brain of PD patients and are activated during the disease progresses. In addition, Hunot et al. reported that CD4+ and CD8+ T lymphocytes infiltrated in the substantia nigra of PD patients, indicating that in addition to central glial activation, peripheral immune cells such as T lymphocytes are involved in mediating neuroinflammation during the pathogenesis of PD. Under physiological conditions, peripheral immune cells are difficult to enter the center due to the presence of the blood-brain barrier (BBB). BBB dysfunction in PD patients means that BBB injury is involved in the development of PD neuroimmune inflammation. Along with the activation and infiltration of these inflammatory cells, many pro-inflammatory cytokines are released such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interferon-γ (IFN-γ) and chemokines. A major pathological feature of PD patients is the formation of lewy body (LB) in dopamine neurons. It has been reported that immunoglobulin G (IgG) is localized on LB of PD patients and forms an immune complex with α-synuclein. It then interacts with the Fcγ receptor-I (FcγR-I) on the surface of microglia to activate microglia and mediate neuroinflammation. In summary, the course of PD is indeed accompanied by the development of immune inflammation, which is characterized by glial cell activation, peripheral immune cell infiltration, immune complex deposition, and the production and release of many pro-inflammatory cytokines and chemokines. Interestingly, even though knocking out cytokine receptors, such as TNFR1 and TNFR2, partially inhibit the activation of inflammatory cells such as microglia but fail to reverse the toxic effects of MPTP on dopamine neurons. These studies suggest that the activation and infiltration of various inflammatory cells interact with various pro-inflammatory cytokines and chemokines secreted by them, mediating the continuous activation and amplification of inflammation, so blocking the expression of a certain cytokine alone cannot be reversed. Therefore, it is necessary to further study the molecular mechanism of immune inflammation in PD and to find the key link that mediates the occurrence and amplification of neuroinflammation, to block the occurrence of neuroinflammation from the source.
Neuroinflammation Research Status
The current research on neuroinflammation focuses on the relationship between neuroinflammation and different diseases. Although neuropathic pain can be caused by a variety of reasons, the basic common mechanism is that the neuroinflammatory response is impaired or affected. This inflammatory response initiates a cascade leading to increased local perfusion, increased capillary permeability, and aggregation and activation of congenital immune cells at the site of tissue damage, irritation or infection, and the resulting inflammatory response environment can activate microglia and astrocytes in the central nervous system. Activated microglia and astrocytes can release various pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1P, interleukin-18 (IL-18), interleukin-6 (IL-6) and inflammatory mediators such as chemokines which are involved in the development and progression of neuropathic pain. In recent years, great progress has been made in the study of glial cell-derived pro-inflammatory cytokines in neuropathic pain states. For neuroinflammatory treatment of Parkinson's disease, non-steroidal anti-inflammatory drugs (NSAIDs) were first proposed for the treatment of Parkinson's disease. In various PD animal models, NSAIDs such as aspirin and acetaminophen inhibit neuroinflammation represented by microglial activation and protect dopamine neurons. Further, in the MPTP model (a model of neural inflammation), it was found that cyclooxygenase-2 (COX-2) is up-regulated in the substantia nigra region, and prostaglandin (PGs) synthesis is increased. NSAIDs exert an inhibitory effect on inflammation by inhibiting COX-2. Therefore, several clinical studies have focused on whether NSAIDs can effectively intervene in PD. However, clinical studies showed that patients taking PD with NSAIDs did not significantly improve the treatment of PD, nor did they reduce the risk of PD. In addition to non-steroidal anti-inflammatory drugs, tetracycline drugs represented by minocycline have also been reported to inhibit neuroinflammation and protect dopamine neurons by inhibiting microglial activation in PD animal models. However, like NSAIDs, a phase II clinical trial in 2008 indicated that PD patients taking minocycline for 3 years did not reverse the progression of Parkinson's disease. Therefore, to date, there have been no effective PD neuroprotective drugs against neuroinflammation. Why can anti-inflammatory drugs successfully protect dopamine neurons in PD animal models and are often frustrated in clinical trials? The reason may be that current patient's substantia nigra dopaminergic neuron death has exceeded 70% in the presence of PD motor symptoms. Even if the inflammation in the brain is inhibited, it is impossible to regenerate more than 70% of the dopamine neurons that have died. It is also impossible to rule out that these drugs are not well inhibited in the human body by dose. Therefore, the current clinical use of anti-inflammatory drugs is not effective. In addition to traditional anti-inflammatory drugs, molecular therapy is also one of the research hotspots of PD therapy. There are also some receptors on the surface of microglia that act to inhibit microglia activation and inhibit neuroinflammation. CD200R is recognized as a receptor that targets anti-inflammatory effects in microglia. It has been found that the up-regulation of CD200R in macrophage patients with Parkinson's disease is impaired, suggesting that its ability to inhibit microglial activation is diminished. In the rat 6-OHDA model, blockade of CD200R exacerbated neuroinflammation and more death of dopamine neurons. These results suggest that CD200-CD200R may also play an anti-inflammatory role in PD. Therefore, overexpression of CD200 in vivo can be studied as a new strategy for PD therapy. CX3CR1 is also a receptor for anti-inflammatory function on the surface of microglia, which binds to a soluble CX3CL1 ligand (Francine) and acts to inhibit inflammation. Studies have shown the use of adeno-associated virus AAV-CX3CL1 in the striatum overexpressing soluble Fractalkine or by continuous administration of soluble Fractalkine in the striatum reversible MPTP model and 6-OHDA model of neuroimmune inflammation and protection of dopamine neurons. In the next step, these molecular therapies are included in clinical studies. The mechanism of PD treatment around the mechanism of neuroinflammation is likely to increase in the future, but the most important thing is to find the optimal time for the treatment of PD with neuroinflammation. The nature of PD is “dopamine neuroselective, progressive death”, and animal models suggest that neuroinflammation also occurs early in the PD course, mediating progressive degeneration of dopamine neurons.
Relationship with Disease
Parkinson's syndrome
Nowadays, it has been confirmed that neuroinflammation plays an important role in the development of Parkinson's disease. Studying the specific mechanism of neuroinflammation in Parkinson's disease will help to use neuroinflammation as a therapeutic target in the future.
References:
- Ransohoff R M, Schafer D, Vincent A, et al. Neuroinflammation: Ways in Which the Immune System Affects the Brain. Neurotherapeutics. 2015, 12(4):896-909.
- Nazem A, Sankowski R, Bacher M, et al. Rodent models of neuroinflammation for Alzheimer's disease. Journal of Neuroinflammation. 2015, 12(1):1-15.
- Lyman M, Lloyd D G, Ji X, et al. Neuroinflammation: the role and consequences. Neuroscience Research. 2014, 79(1):1-12.
- Surendranathan A, Rowe J B, O'Brien J T. Neuroinflammation in Lewy body dementia. Parkinsonism & Related Disorders. 2015, 21(12):1398-1406.
- Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson's disease and its potential as therapeutic target. Translational Neurodegeneration. 2015, 4(1):19.
- Whats New
- Shopping
- Wellness
- Sports
- Theater
- Religion
- Party
- Networking
- Music
- Literature
- Art
- Health
- Jocuri
- Food
- Drinks
- Fitness
- Gardening
- Dance
- Causes
- Film
- Crafts
- Other/General
- Cricket
- Grooming
- Technology

