Neurodegenerative Diseases
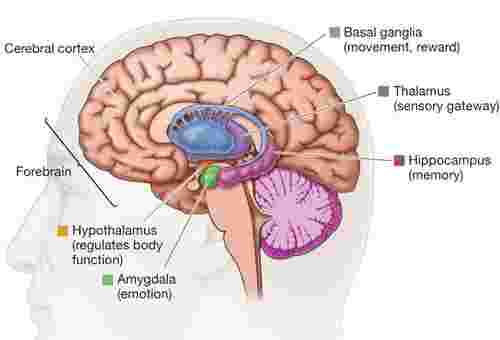
Neurodegenerative diseases overview
Neurodegenerative diseases are caused by the loss of neurons and myelin, which deteriorate over time and cause dysfunction. It can be divided into acute neurodegenerative diseases and chronic neurodegenerative diseases. The former mainly includes cerebral ischemia (CI), brain injury (BI), and epilepsy; the latter includes Alzheimer's disease (AD) and Parkinson's disease (PD), Huntington's disease (HD), amyotrophic lateral sclerosis (ALS), different types of spinocerebellar ataxia (SCA), Pick disease, etc. There are two main manifestations of brain pathological changes in this type of disease; one is the loss of many neurons caused by apoptosis; the other is that the nervous system does not have a significant decrease in the number of cell bodies. With the deepening of research on neurodegenerative diseases, more and more factors are found to be related to the occurrence and development of neurodegenerative diseases.
Mechanism of Neurodegenerative Diseases
Many factors are involved in the neurodegenerative diseases, such as oxidative stress. Oxidative stress is caused by excessive production of free radicals and/or indiscriminate clearance, and the imbalance between oxidation and anti-oxidation in the body, and the cells and tissues of the body is damaged. In recent years, the theory of oxidative stress has received increasing attention, and oxidative damage of nerve tissue has been found in neurodegenerative diseases such as AD, PD, and ALS. In the nervous system, the metabolism of excitatory amino acids and neurotransmitters is the source of oxygen free radicals; at the same time, various causes (such as Aβ aggregation, and calcium imbalance.) can damage mitochondria, cause mitochondrial dysfunction, and release many reactive oxygen species (ROS). The pathological features of AD include senile plaques formed by the accumulation of β-amyloid (Aβ) in the nerve cells (SP) and neurofibrillary tangles (NFT) formed by abnormal aggregation of Tau proteins in cerebral neurons. There are many reasons for brain tissue damage caused by free oxygen free radicals. First, the cerebral vascular endothelium suffers from oxidative stress damage, causing cerebrovascular dysfunction. Second, oxygen free radicals bind to Aβ protein and activate God protein. The receptor for glycosylation end products on the cells and microglia causes peroxidative damage and even apoptosis of the cells. In addition, free radicals interact with metal ions and aggravate brain tissue damage in a variety of forms, such as oxidized Fe3+, which promotes phosphorylation of Tau protein and accumulation of Tau protein. Studies have shown that the increase in free radical production due to functional defects in the antioxidant defense system plays a major role in the pathogenesis of PD. For example, glutathione (GSH) is an important free radical scavenger in the body. In the substantia nigra of PD patients, GSH is greatly reduced, and the amount of reduction is positively correlated with the severity of PD. In addition, mitochondrial dysfunction also induces oxidative damage. Mitochondria are present in the cytoplasm of eukaryotes and organelles containing extranuclear genetic material and having a bilayer membrane structure. It is the most basic unit of energy produced in cells and contains the major enzymes in the electronic respiratory chain. The respiratory chain enzyme component on the mitochondrial inner membrane converts the metabolites of carbohydrates, proteins, and lipids into CO2 and water by participating in the oxidative phosphorylation process, and producing a large amount of adenosine triphosphate (ATP), which supplies energy to the cells. PD was the first neurodegenerative disease associated with mitochondrial dysfunction. Studies have shown that in the substantia nigra pars compacta, striatum and platelets of PD patients, the function of mitochondrial respiratory chain complex I is reduced by 30%, and nigra pars compacta, mitochondrial respiratory chain complex IV and tricarboxylic acid cycle rate-limiting enzyme -ketoglutarate dehydrogenase activity is reduced. In maternal-inherited familial PD patients, the mitochondrial respiratory chain complex I function is diminished in children with pedigrees, accompanied by increased ROS and increased antioxidant enzyme activity. In addition, enhanced lipid peroxidation decreased GSH consumption, and increased oxidative stress was also found in the substantia nigra pars compacta. The study found that there are mtDNA defects and oxidative phosphorylation abnormalities in the brain of AD patients. Polymerase chain reaction (PCR) and blot hybridization revealed mtDNA breaks, base deletions and missense mutations in brain tissue of patients with the sporadic AD. Electron microscopic observation confirmed that the number of mitochondria increased, the structure was abnormal, and lamellar bodies and crystalline inclusion bodies appeared. In addition, AD patients with neuronal mitochondrial dysfunction will lead to the insufficient energy supply of neurons and release a large amount of ROS, induce oxidative stress damage and imbalance of calcium regulation, and ultimately trigger neuronal apoptosis. The most important excitatory neurotransmitters in the central nervous system, mainly mediated by glutamate receptors, fulfill their numerous functions in the brain. Activation of glutamate receptors, in addition to rapid excitatory synaptic transmission, synaptic plasticity can also regulate neurotransmitter release. However, if the concentration of glutamate in the intercellular space is too high, it will be toxic to neurons, leading to neuronal degeneration, aging, and death. This excitatory toxic effect of glutamate is closely related to the occurrence and development of various neurodegenerative diseases and is one of the important mechanisms leading to the death of nerve cells in neurodegenerative diseases. Glutamate receptors play an important role in neurodegenerative diseases, participating in synaptic transmission under normal conditions and neuroprotective effects by partial metabotropic glutamate receptors when synaptic activity is enhanced. Moreover, there are the excitotoxicity mediated by ionotropic glutamate receptors. Excitotoxicity refers to the neurotoxic effects of excessive excitatory amino acids (EAA) and involves mechanisms that are mediated by N-methyl-D-aspartate (NMDA) receptor over-excitation, which is characterized by the persistent Ca2+ influx and delayed neuronal damage. Due to the large amount of Ca2+ influx and the rapid accumulation of Ca2+ in the mitochondria, mitochondrial function loss can be induced. In addition, the activity of nitric oxide synthase can also be increased in the process, and the increase of NO induces a high toxic effect. In addition, beta-amyloid precursor protein and Tau protein can inhibit the uptake of extracellular glutamate, and this inhibition will lead to increased extracellular glutamate levels, resulting in excitotoxic effects.
Immune Inflammation
The inflammatory hypothesis of degenerative disease has only recently been raised. There is a lot of evidence that inflammation plays an important role in the pathogenesis of AD. The innate immune system is a natural immune defense function that is formed during germline development and evolution. Compared with another specific immune response of the body, it can respond quickly to various harmful substances to protect tissue and organ. The activation of the innate immune system itself is a double-edged sword. Harmful substances (such as aggregated forms of Aβ) cause long-term and uncontrollable stimuli that activate the innate immune system and cause damage to the brain. The main hallmark of brain inflammation is the activation of glial cells, especially microglia. Microglia-mediated chronic inflammatory response plays an important role in the development and progression of neurodegenerative diseases. Activation of pro-inflammatory cytokines and free radicals released by microglia initiate or amplify the neuronal damage response, while neuronal damage, in turn, activates microglia, forming a vicious circle. There are abundant activated microglia in the brain of AD patients. Activated microglia can produce neurotoxic substances such as complement protein, inflammatory factors, and acute phase reactants, thereby activating immune inflammatory reactions and producing neurotoxicity. Clinical studies have found that long-term use of non-steroidal anti-inflammatory drugs can reduce the progression of the AD. Studies have shown that the entry of Ca2+ into the cell from outside the cell leads to the destruction of calcium balance, which is the central link of cell death. Cellular Ca2+ overload plays an important role in PD, HD, and ALS diseases. For example, the Ca2+ antagonist nimodipine blocks the development of PD caused by MPTP in primates and mice. MPTP neurotoxicity disrupts the stability of Ca2+, and Ca2+, as a trigger for apoptosis, may regulate signal transmission by activating Ca2+-dependent endonucleases or by other effects. The Na+/Ca2+ exchange in the brain of AD patients is significantly higher than that of normal people. The decrease of serum Ca2+ concentration in patients can increase the secretion of parathyroid hormone and activate the adenylate cyclase through the protein pathway to increase the concentration of intracellular cyclic adenosine monophosphate, to promote calcium influx, induce the increase of Ca2+ in brain cells, and then degrade dementia by destroying mitochondrial function and other means to reduce cell energy metabolism. Decreased blood Ca2+ may also affect cell membrane permeability and cell-cell interactions, thereby interfering with the growth and development of nerve cells, especially the metabolism of free radicals.
Neurodegenerative Diseases Research Status
Neurodegenerative diseases have become a major social problem that threatens the health and longevity of older people. Autophagy is a living phenomenon in eukaryotic cells. It can involve in normal cell growth, development and many physiological and pathological processes. It can eliminate excess or damaged organelles and proteins in cells, participate in antigen presentation and resist microbial intracellular infection. It plays an important role in protein metabolism balance and intracellular environmental homeostasis. It has a positive effect on neurodegenerative diseases, tumors, cardiomyopathy, pathogenic microorganisms from invading infections, preventing aging and prolonging life. Autophagy is the formation of autophagosomes by the cells in a monolayer or bilayer membrane and are then transported to lysosomes to form autophagosomes and digest and degrade various enzymes to achieve the metabolic needs of the cells themselves. Most of the neurodegenerative diseases have obvious characteristics of abnormal accumulation of proteins, which have toxic effects on neurons and ultimately lead to neuronal death. The role of autophagy in neurodegenerative diseases has received increasing attention in recent years. The intervention of autophagy to intervene in neurodegenerative diseases has broad research and application prospects. A variety of aberrantly expressed miRNAs play an important role in the progression of neurodegenerative diseases. miRNAs are of great value in the detection, diagnosis, and treatment of neurodegenerative diseases. Specific miRNAs provide new targets and ideas for drug development, clinical diagnosis and treatment of neurodegenerative diseases. However, there are still many problems before miRNA technology is widely used in clinical diagnosis and treatment. With the maturity of RNA interference technology, the in vivo mechanism of action, safety, targeting, effectiveness, and specificity of miRNAs will be solved one by one. The research results of miRNAs will be more widely used in gene therapy of diseases.
References:
- Newberg A B, Serruya M, Wintering N, et al. Meditation and neurodegenerative diseases. Annals of the New York Academy of Sciences. 2014, 1307(1):112-123.
- Sharma M, Tiwari M, Tiwari R K. Hyperhomocysteinemia: Impact on Neurodegenerative Diseases. Basic & Clinical Pharmacology & Toxicology. 2015, 117(5):287-296.
- Tellone E, Galtieri A, Russo A, et al. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxidative Medicine & Cellular Longevity. 2015, 2015(6415):1-14.
- Ariga H. Common mechanisms of onset of cancer and neurodegenerative diseases. Biological & Pharmaceutical Bulletin. 2015, 38(6):795-808.
- Whats New
- Shopping
- Wellness
- Sports
- Theater
- Religion
- Party
- Networking
- Music
- Literature
- Art
- Health
- Giochi
- Food
- Drinks
- Fitness
- Gardening
- Dance
- Causes
- Film
- Crafts
- Other/General
- Cricket
- Grooming
- Technology

