-
Feed de Notícias
- EXPLORAR
-
Páginas
-
Grupos
-
Eventos
-
Blogs
-
Marketplace
-
Fóruns
-
Jogos
Bacterial Secretion System Immunology Reagents
Overview of bacterial secretion system
Protein secretion, the transport of proteins from the cytoplasm into other parts of the cell, the environment, and/or other bacteria or eukaryotic cells, is one of the essential functions of a prokaryotic cell. Prokaryotes have developed a number of transporting proteins between locations which are largely involved in the help of dedicated protein secretion systems. Protein secretion systems are important for the growth of bacteria and are useful in a lot of processes. It has been found that some secretion systems are almost in all bacteria and they will secrete a wide variety of substrates, while others have been found in just a few bacterial species and are involved to secrete only one or a few proteins. In certain cases, bacterial pathogens use these dedicated secretion systems to manipulate the host and build a replicative niche. Other times, these secretion systems will make use of an environmental niche to assist bacteria to compete with nearby microorganisms. Several different classes of bacterial secretion systems exist, and their designs differ based on the number of transmembranes in the protein substrate.
Secretion across the cytoplasmic membrane
There are two pathways which are the bacterial secretion systems most commonly used to transport proteins across the cytoplasmic membrane: the general secretion (Sec) and twin arginine translocation (Tat) pathways. These two pathways are considered the most highly conserved mechanisms of protein secretion, and have been identified in all domains of life (bacteria, archaea, and eukarya). In bacterial, Sec and Tat pathways exist in the periplasm or the inner membrane, and transport most proteins. Proteins in unfolded state are translocated by the Sec pathways primarily. This system is composed of three parts: a protein targeting component, a motor protein, and a membrane integrated conducting channel, which is called the SecYEG translocase. In contrast to the Sec pathway, the Tat pathway mainly secretes folded protein. This pathway is important because not all proteins can be secreted in their unfolded state.
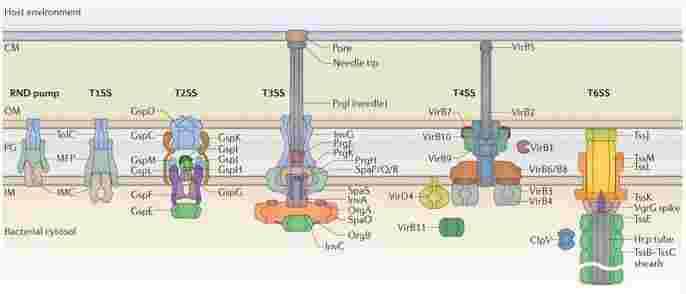
Protein secretion by gram-negative bacteria
Dedicated secretion systems are relied on by a number of Gram-negative bacteria to transport virulence proteins outside of the cell and, in some times, directly into the cytoplasm of a eukaryotic or prokaryotic target cell. It is a challenge for Gram-negative bacteria to secrete extracellular proteins, because these proteins must cross two or even three phospholipid membranes in order to finally arrive at their destination. Some secreted proteins will cross these membranes in two separate steps, where they are first delivered to the periplasm via the Sec or Tat secretion systems, and are then transferred across the outer membrane by a second system, which is known as Sec- or Tat-dependent protein secretion. In addition, a process known as Sec- or Tat-independent protein secretion is used to secrete many other proteins through channels that span both the inner and outer bacterial membranes. These systems in the Gram-negative bacteria are numbered from Type I to Type VI, with each system supporting a specific subset of proteins. β-barrel channels are involved in these in systems which form a ring in the outer membrane of the bacterial cell, but show up a fair amount of diversity in their structures and mechanistic functions otherwise.
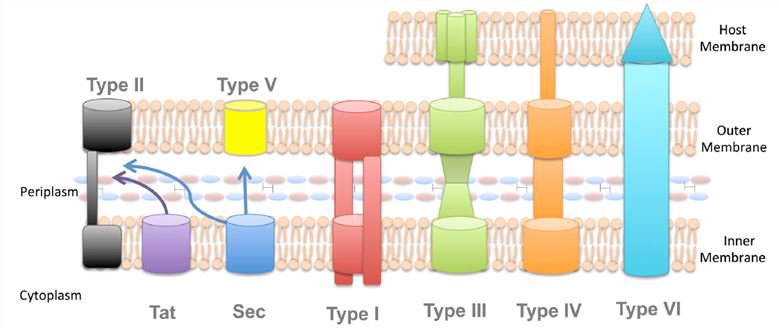 Figure 1. Secretion systems in Gram-negative bacteria
Figure 1. Secretion systems in Gram-negative bacteria
Protein secretion by gram-positive bacteria
In contrast with the Gram-negative bacteria which possess two phospholipid membranes, Gram-positive bacteria contain only one lipid bilayer and are covered by a very thick cell wall which is considerably thicker than that of Gram-negative bacteria. In addition, there are some species of Gram-positive bacteria which contain a cell wall heavily modified by lipids called a mycomembrane. Considering these differences in basic cell structure, it is obvious that the mechanisms of extracellular protein secretion differ between the Gram-positive bacteria and the Gram-negative bacteria. Similar to the Gram-negative organisms, Gram-positive bacteria rely to both the Tat and Sec pathways to transport proteins across the cytoplasmic membrane. However, in most cases, it is not sufficient to deliver the proteins to their destination. Also similar to their Gram-negative counterparts, proteins which assist Gram-positive pathogens in survival during infection of a mammalian host are expressed on their outer surfaces. These proteins must embed themselves into the Gram-positive bacteria cell wall so as to be retained on the outer surface of the bacterium. In order to achieve this function, a class of enzymes, call sortases, is encoded by Gram-positive bacteria, which covalently make the proteins attached to the cell wall following secretion across the cytoplasmic membrane. These sortases vary in their specificity, for example, “housekeeping” sortases, SrtA, can attach as many as 40 proteins to the cell wall, while other sortases are unique for only one or two proteins.
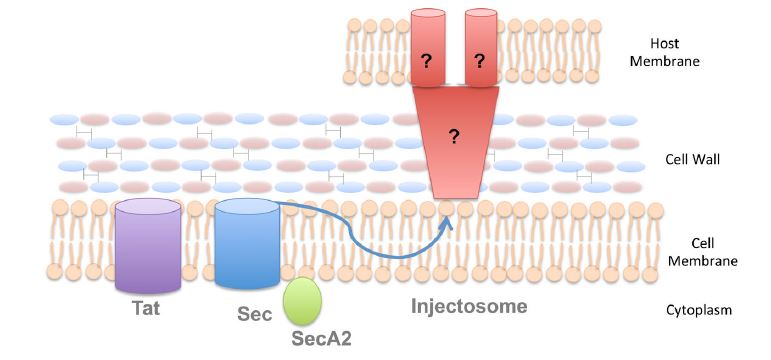
Figure 2. Secretion systems in Gram-positive bacteria
References:
- Green E R, Mecsas J. Bacterial Secretion Systems: An Overview.Microbiol Spectr, 2016, 4(1).
- Natale P, Bruser T, Driessen AJ. Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane--distinct translocases and mechanisms. Biochim Biophys Acta. 2008; 1778(9):1735–56.
- Whats New
- Shopping
- Wellness
- Sports
- Theater
- Religion
- Party
- Networking
- Music
- Literature
- Art
- Health
- Jogos
- Food
- Drinks
- Fitness
- Gardening
- Dance
- Causes
- Film
- Crafts
- Other/General
- Cricket
- Grooming
- Technology

